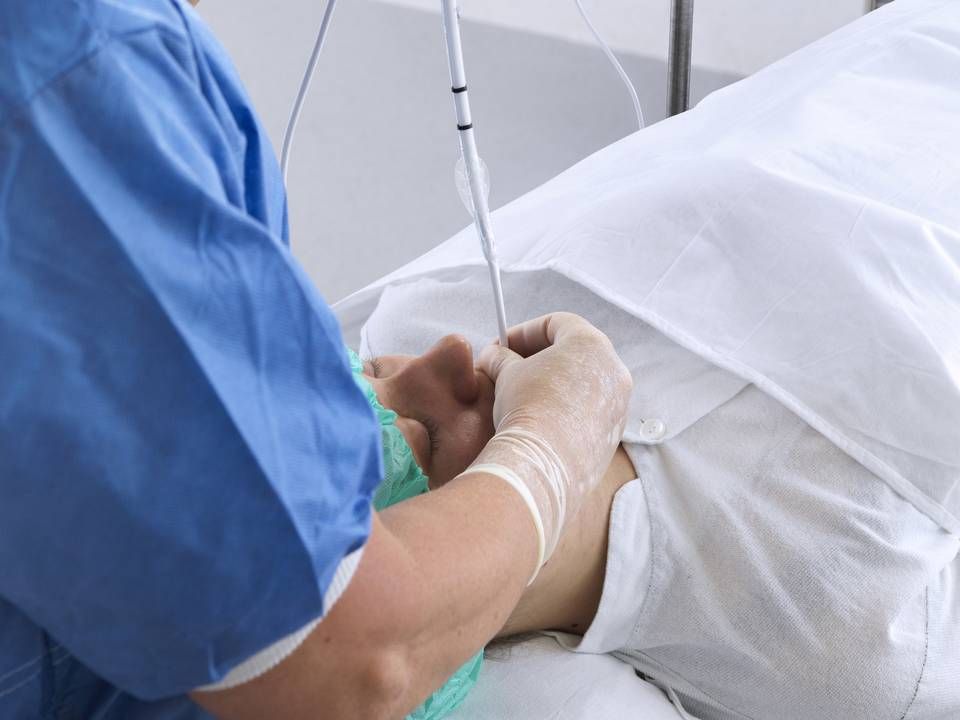
Ambu relaunches formerly recalled product

It has taken Ambu nearly a year to tighten the safety of its VivaSight 2 DLT product, which is a tube and camera to monitor lung ventilation.
Read the whole article
Get access for 14 days for free. No credit card is needed, and you will not be automatically signed up for a paid subscription after the free trial.
With your free trial you get:
Get full access for you and your coworkers
Start a free company trial todayRelated articles
Ambu recalls single-use product one year after launch
For subscribers
FDA approves new Ambu product
For subscribers