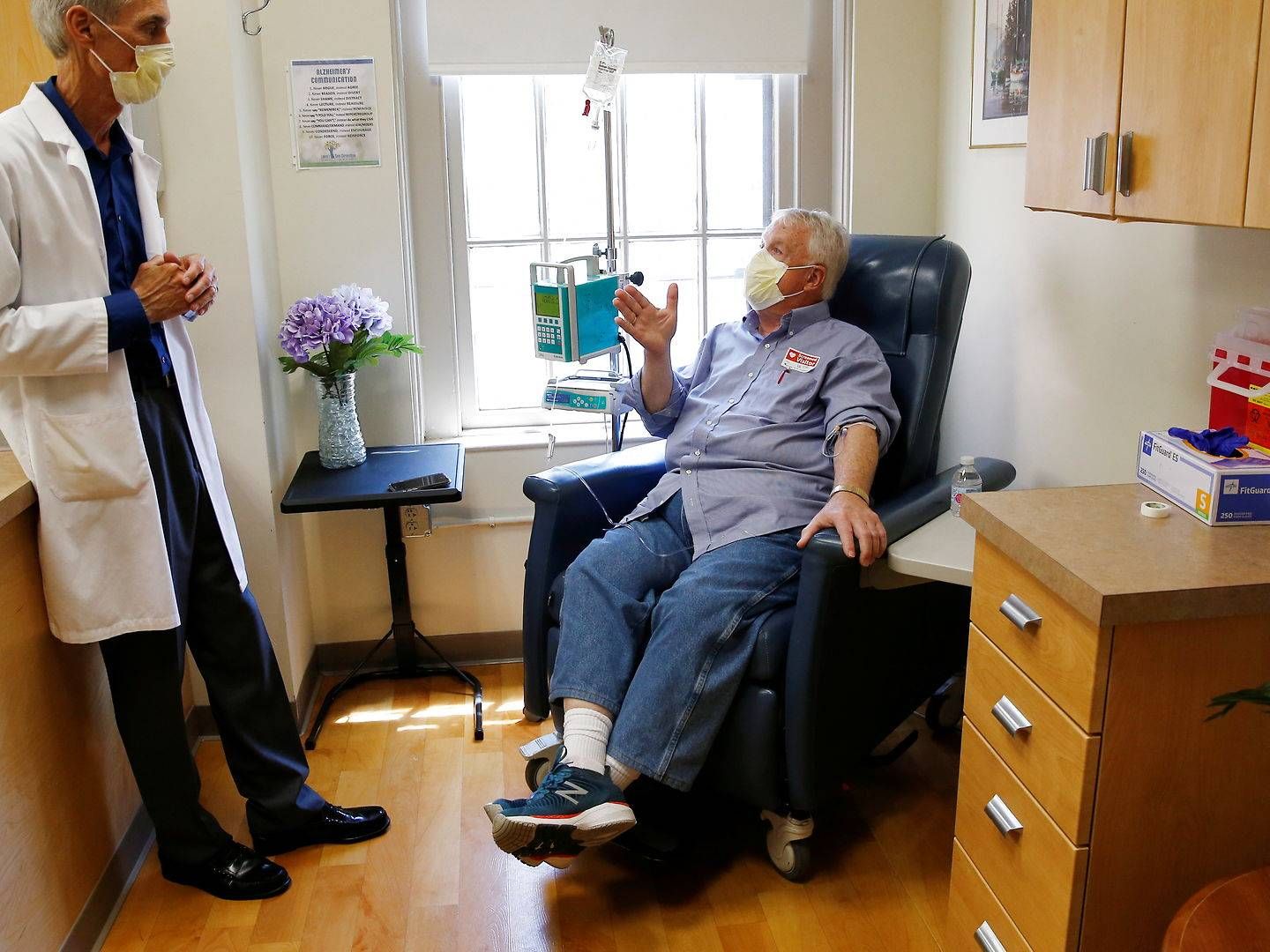
Eisai and Biogen begin application process for second Alzheimer's drug

Eisai and Biogen are now making another attempt to tackle Alzheimer's disease, initiating a "rolling submission" process at the US Food and Drug Administration (FDA) of the drug lecanemab, the companies inform in a press release.
Read the whole article
Get access for 14 days for free. No credit card is needed, and you will not be automatically signed up for a paid subscription after the free trial.
With your free trial you get:
Get full access for you and your coworkers
Start a free company trial todayRelated articles
Biogen partner calls for global Alzheimer's effort
For subscribers