Lundbeck to measure brain with microscopic camera in new partnership
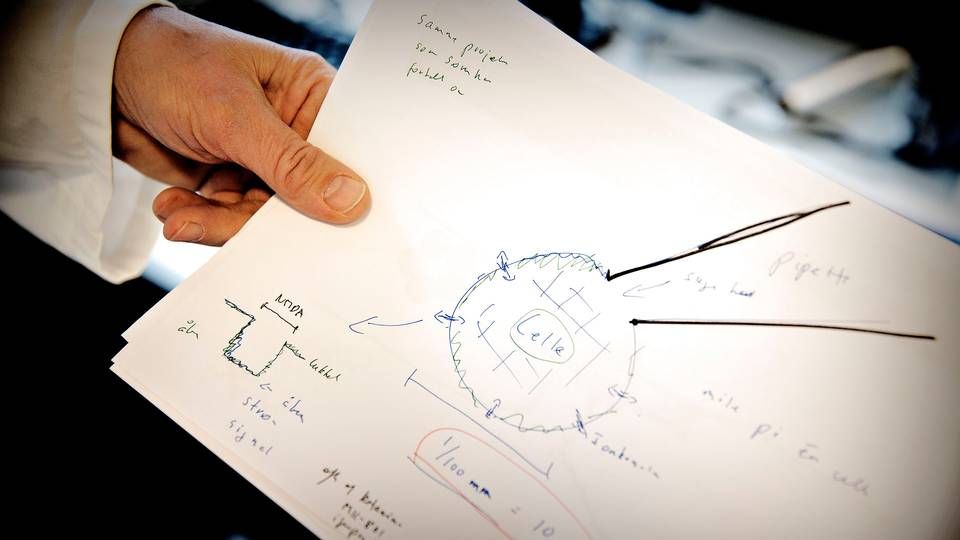
The US Food & Drug Administration's recent approval of Biogen's Alzheimer drug, Aduhelm, has sparked debate and controversy over how to measure the efficacy of medications against psychiatric and neurological disorders.
Read the whole article
Get access for 14 days for free. No credit card is needed, and you will not be automatically signed up for a paid subscription after the free trial.



.jpg)























