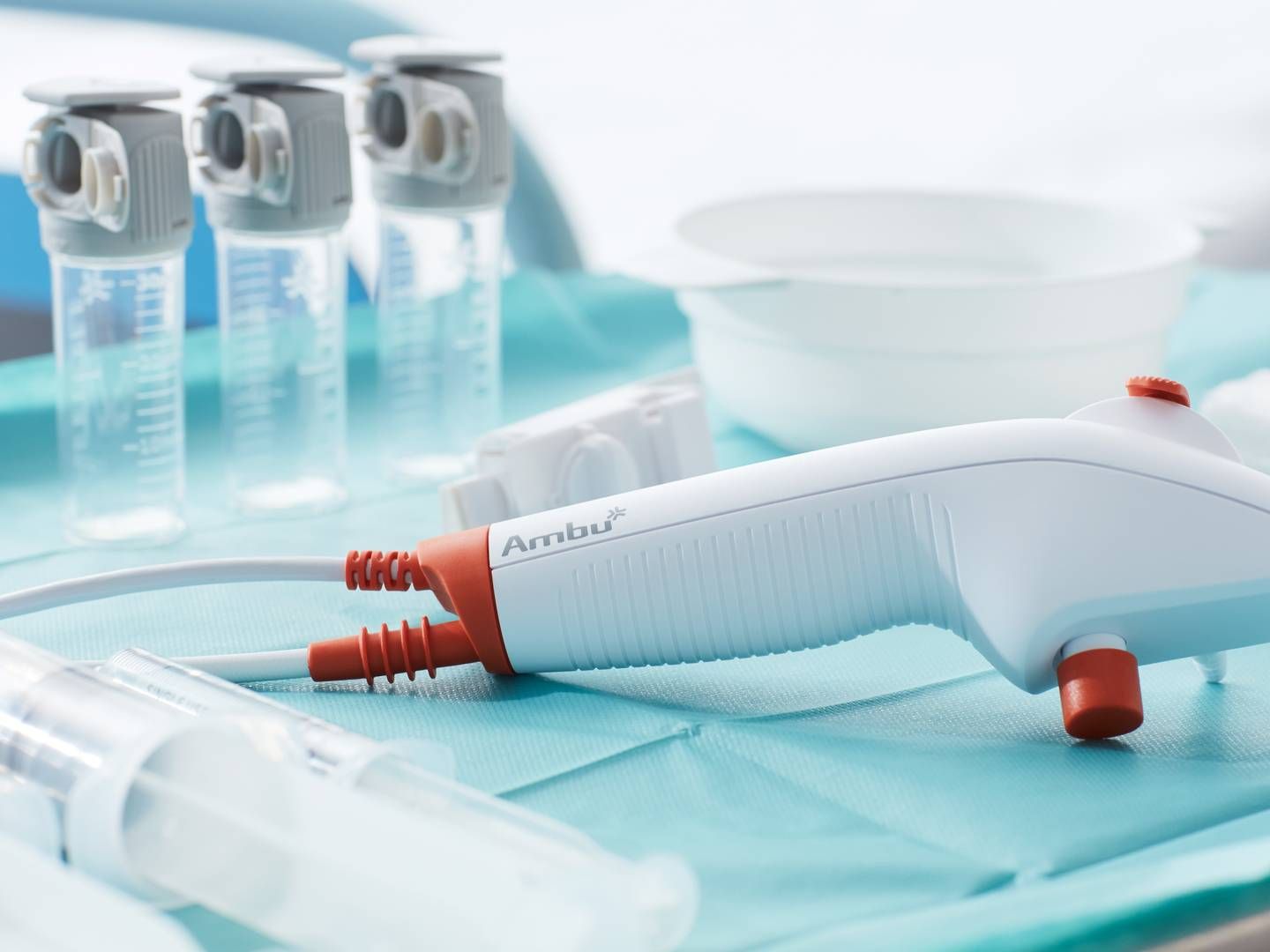
FDA clears Ambu's new bronchoscope generation

After obtaining the EU’s CE mark for the Ascope 5 Broncho device in May, medtech firm Ambu can now also launch in the US, according to a Tuesday press release.
Read the whole article
Get access for 14 days for free. No credit card is needed, and you will not be automatically signed up for a paid subscription after the free trial.
With your free trial you get:
Get full access for you and your coworkers
Start a free company trial todayRelated articles
Ambu's stock hits lowest price in five years
For subscribers
Ambu recalls single-use product one year after launch
For subscribers