Ambu gets recommendation from FDA, analyst doubts effect
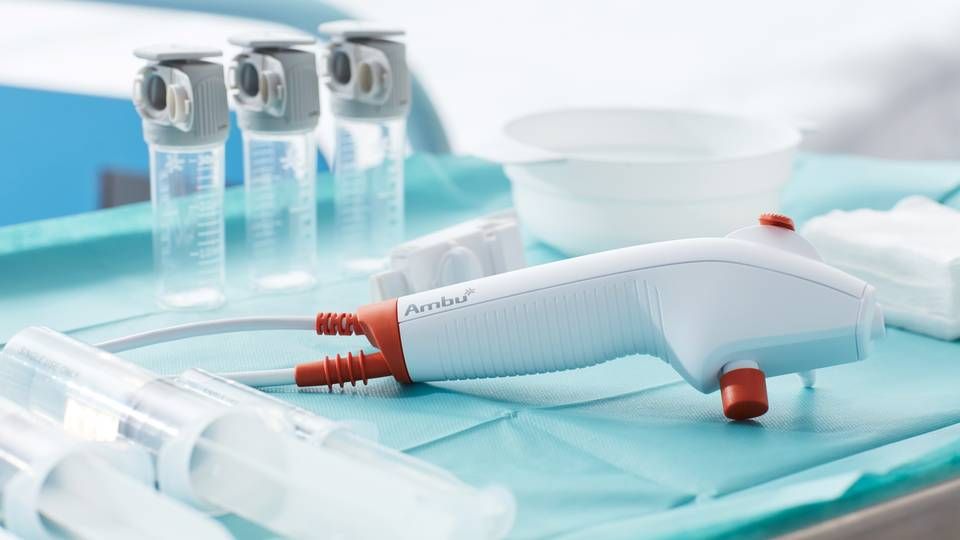
On Friday, the US Food and Drug Administration (FDA) recommended that doctors at US hospitals use single-use bronchoscopes instead of reusable scopes – one year after the American Lung Association gave the same advice.
Read the whole article
Get access for 14 days for free. No credit card is needed, and you will not be automatically signed up for a paid subscription after the free trial.
With your free trial you get:
Get full access for you and your coworkers
Start a free company trial todayRelated articles
FDA recommends single-use bronchoscopes
For subscribers
FDA approves new Ambu product
For subscribers

.jpg)























