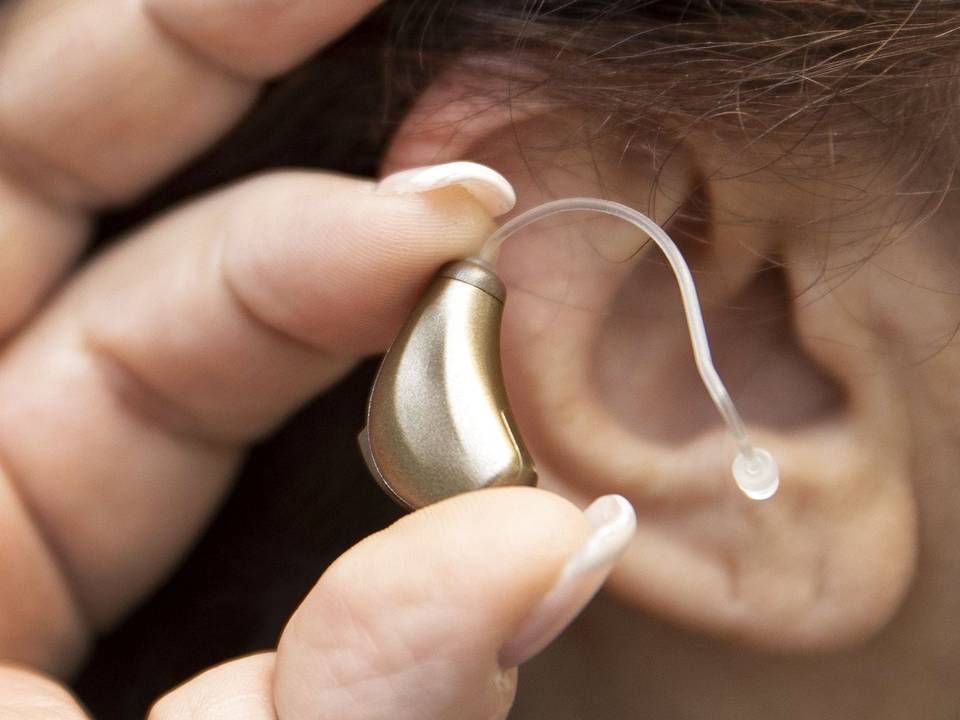
Audientes accelerates preparations for US market entry after OTC market news

The US, which is indisputably the world's biggest single market for hearing aids, is changing its laws regarding the sales of non-prescription devices, with cheaper products being able to hit shelves in September of next year at the latest.
Read the whole article
Get access for 14 days for free. No credit card is needed, and you will not be automatically signed up for a paid subscription after the free trial.