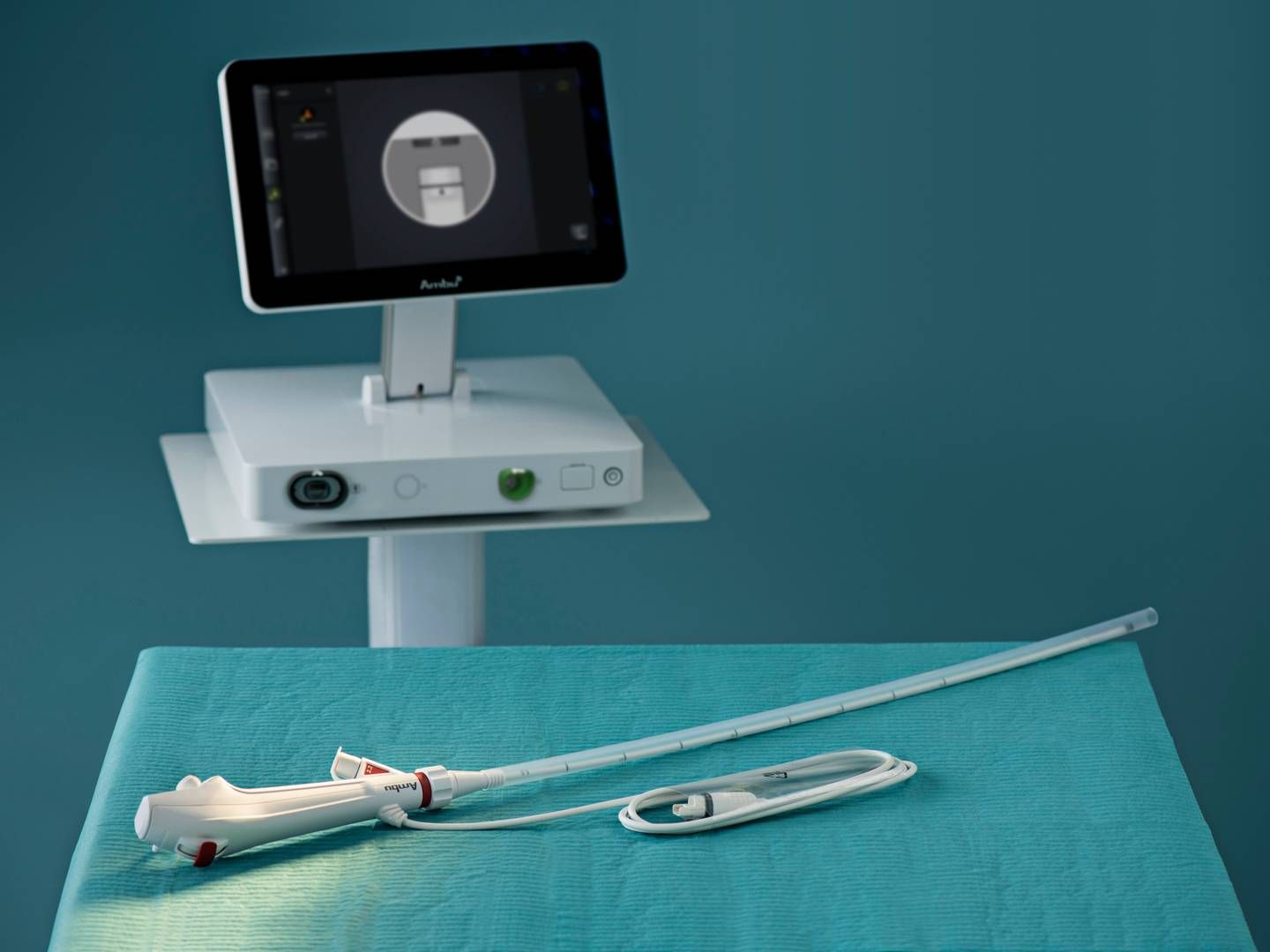
Ambu receives FDA clearance for new generation of endoscopy screens
Ambu’s new generation of Aview endoscopy monitors are now available for sale in North America after the Danish medtech company received clearance for the product from the US Food and Drug Administration (FDA).
Read the whole article
Get access for 14 days for free. No credit card is needed, and you will not be automatically signed up for a paid subscription after the free trial.
With your free trial you get:
Get full access for you and your coworkers
Start a free company trial todayRelated articles
Ambu begins European launch of new product after CE marking
For subscribers