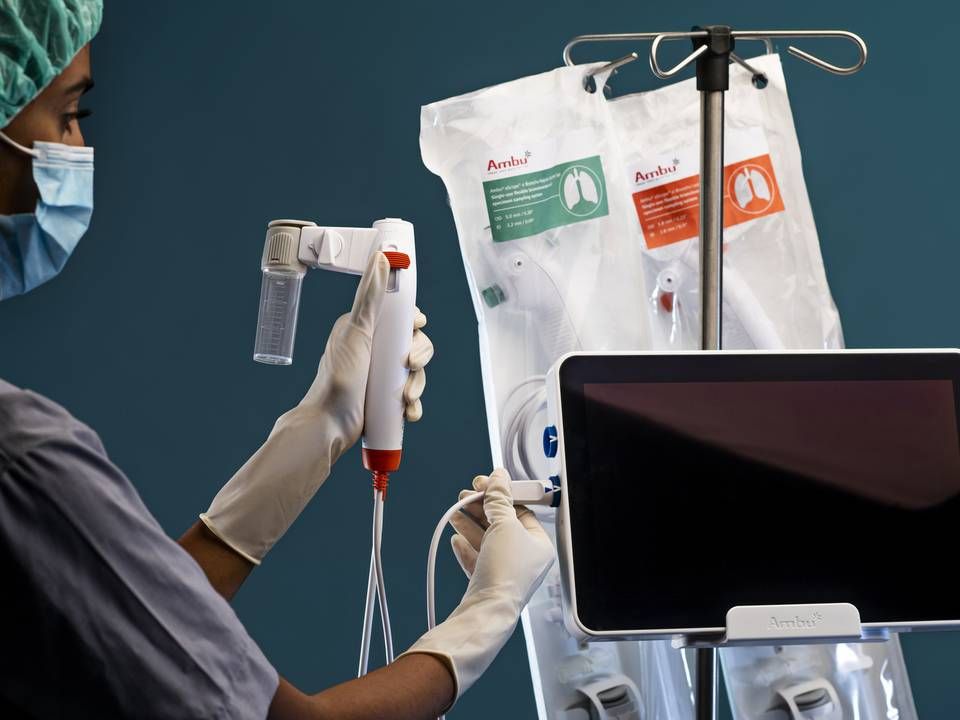
Single-use scopes take center stage at Ambu as core business areas recede
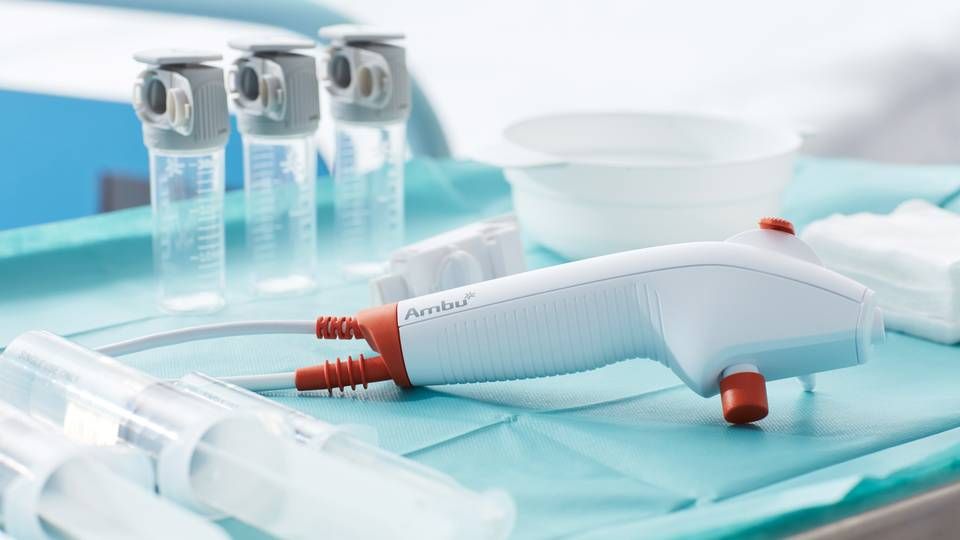
Medtech company Ambu's year has been somewhat of a mixed bag.
Read the whole article
Get access for 14 days for free. No credit card is needed, and you will not be automatically signed up for a paid subscription after the free trial.