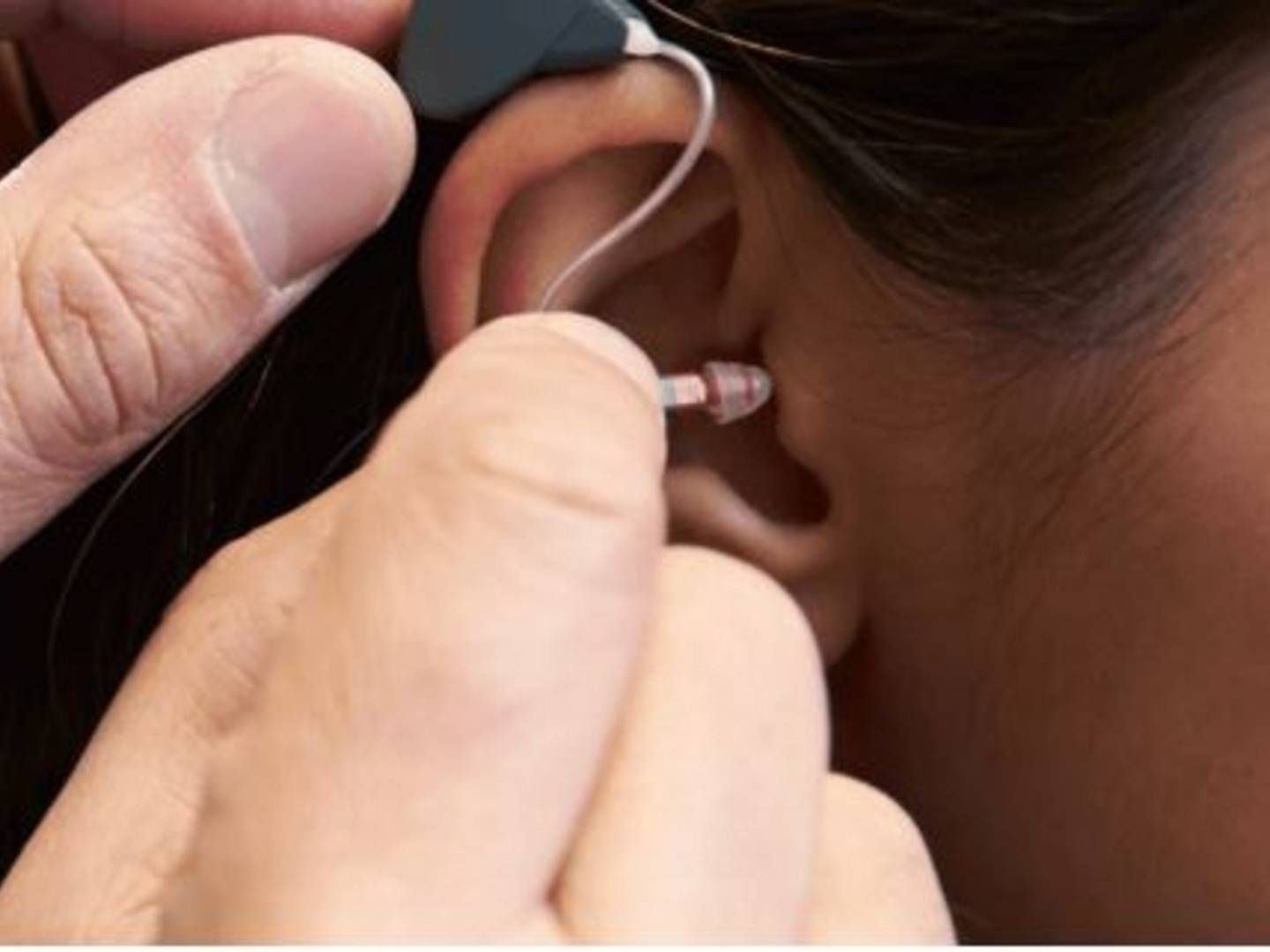
Demant's surgical division loses president of 13 years

Hearing group Demant is going on a hunt for a new president for its surgical division, Oticon Medical, which has marketed hearing implants and bone-anchored solutions since the company’s establishment in 2008.
Read the whole article
Get access for 14 days for free. No credit card is needed, and you will not be automatically signed up for a paid subscription after the free trial.
With your free trial you get:
Get full access for you and your coworkers
Start a free company trial todayRelated articles
Hearing firms urge FDA to increase OTC safety requirements
For subscribers