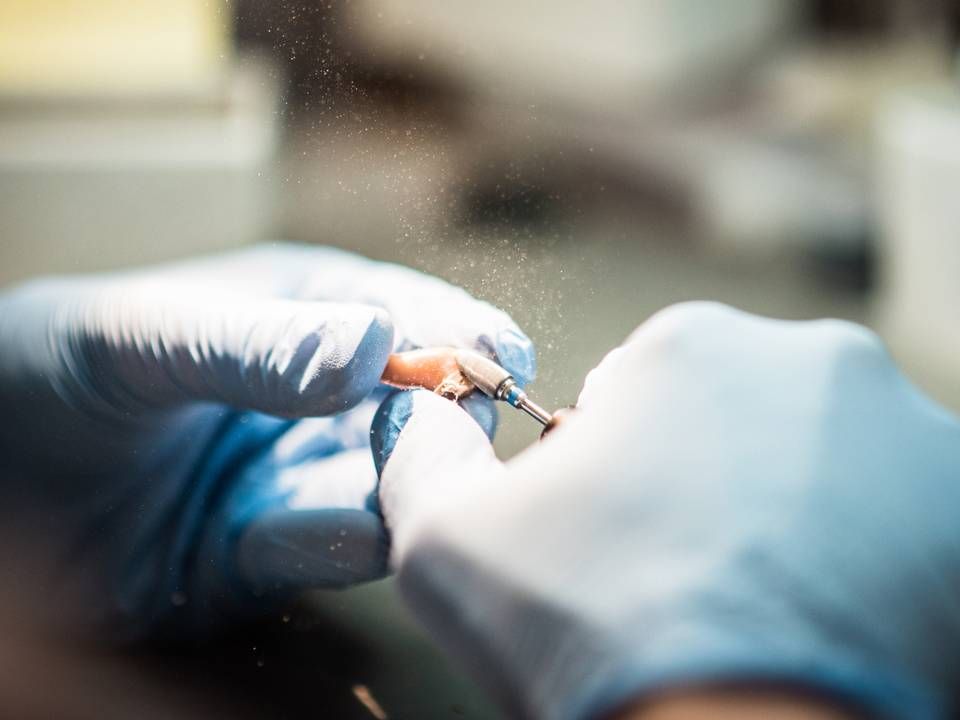
French reforms and US vaccinations benefit Demant in corona-struck times

Demant has raised its guidance for 2021 based on looking back on the first three months, rather than ahead to the next nine, says Søren Nielsen, CEO of Demant, to MedWatch.
Read the whole article
Get access for 14 days for free. No credit card is needed, and you will not be automatically signed up for a paid subscription after the free trial.
With your free trial you get:
Get full access for you and your coworkers
Start a free company trial todayRelated articles
WSA ready to launch new Signia product platform
For subscribers
Demant raises financial guidance for 2021
For subscribers
Analyst predicts that covid-19 will still affect Demant
For subscribers