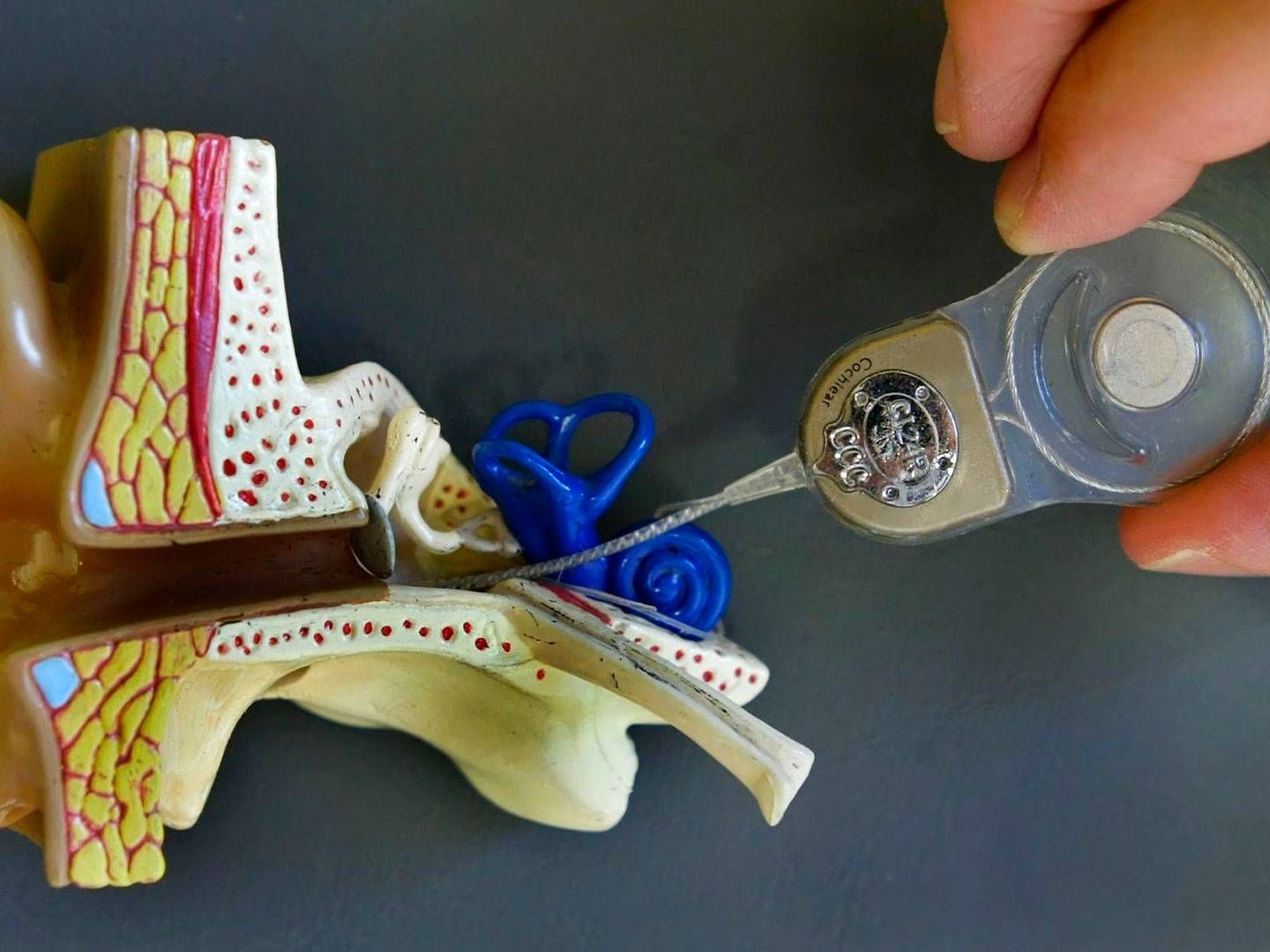
Sonova receives FDA approval for new technology

Swiss hearing aid firm Sonova is launching two new sound processors for adults and children who use cochlear implants, the firm announced on Thursday morning.
Read the whole article
Get access for 14 days for free. No credit card is needed, and you will not be automatically signed up for a paid subscription after the free trial.
With your free trial you get:
Get full access for you and your coworkers
Start a free company trial todayRelated articles
Demant launches new flagship product
For subscribers