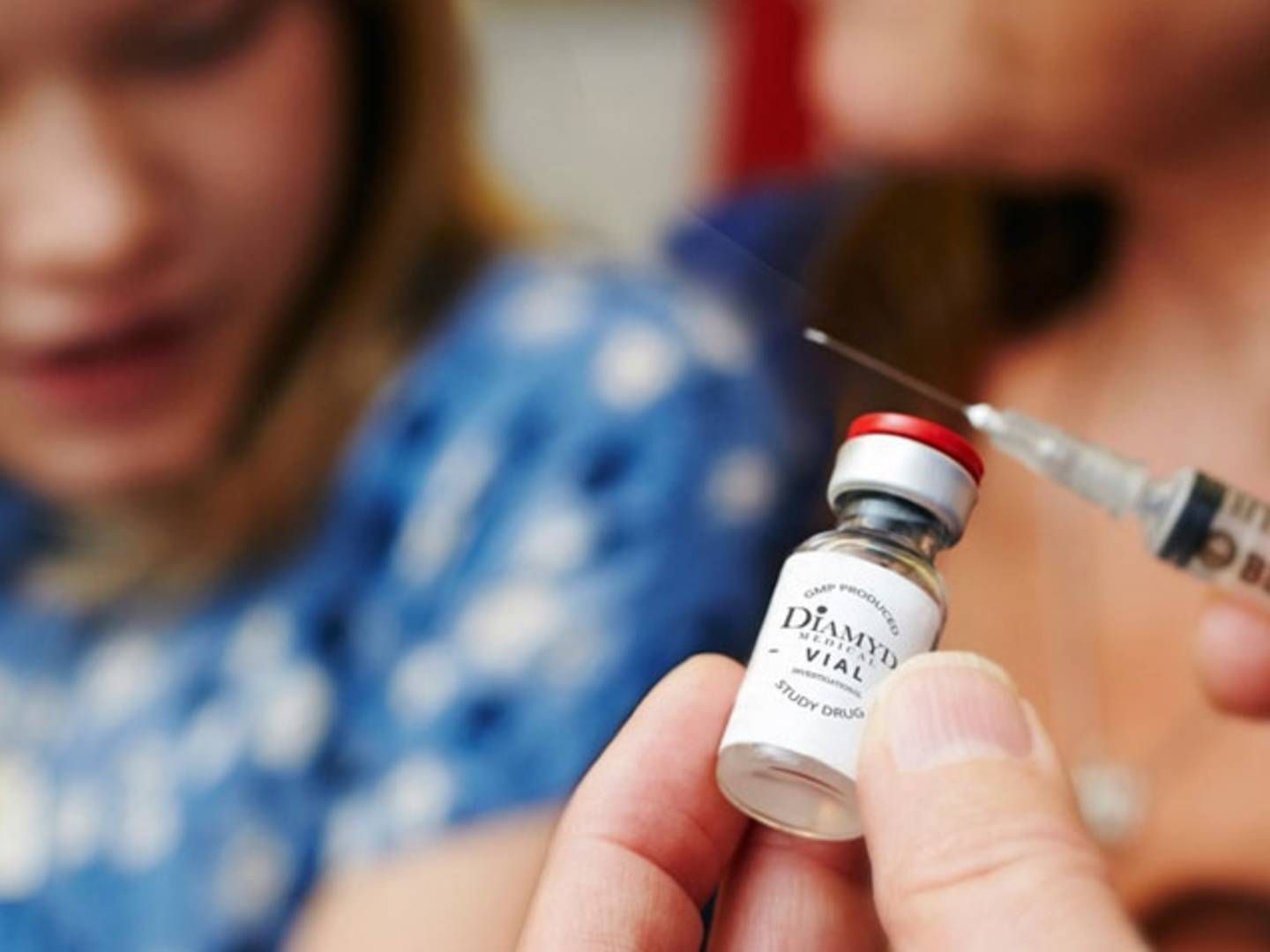
FDA green-lights Diamyd Medical's paused diabetes vaccine study
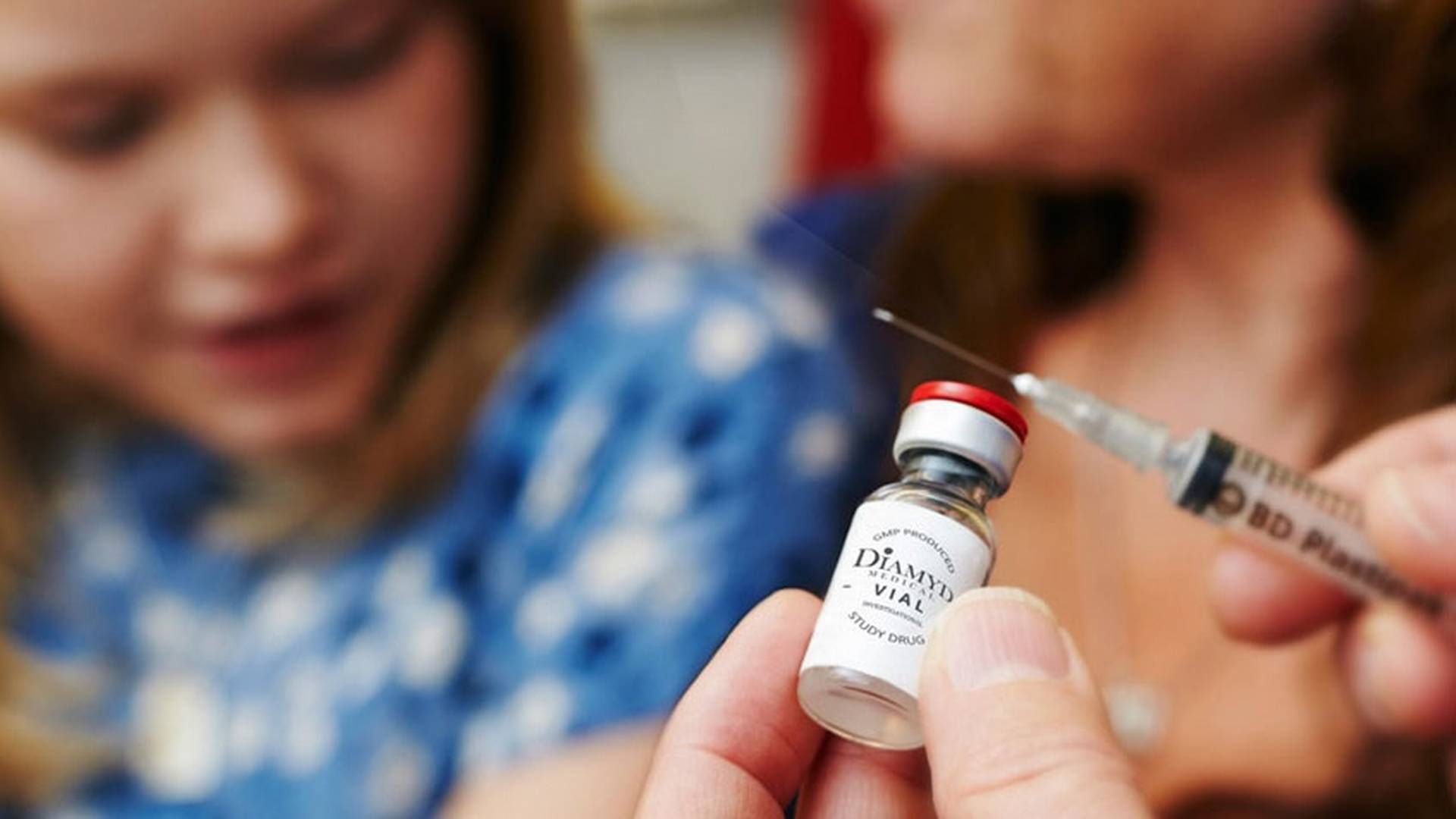
Diamyd Medical surged 20% in Swedish trading following news that the US Food and Drug Administration (FDA) has allowed the biotech company to resume a phase III study of a vaccine for type 1 diabetes patients.
Read the whole article
Get access for 14 days for free. No credit card is needed, and you will not be automatically signed up for a paid subscription after the free trial.
With your free trial you get:
Get full access for you and your coworkers
Start a free company trial todayRelated articles
Diamyd Medical meets study goal with diabetes vaccine
For subscribers
FDA pauses Swedish diabetes company's phase III trial plans
For subscribers