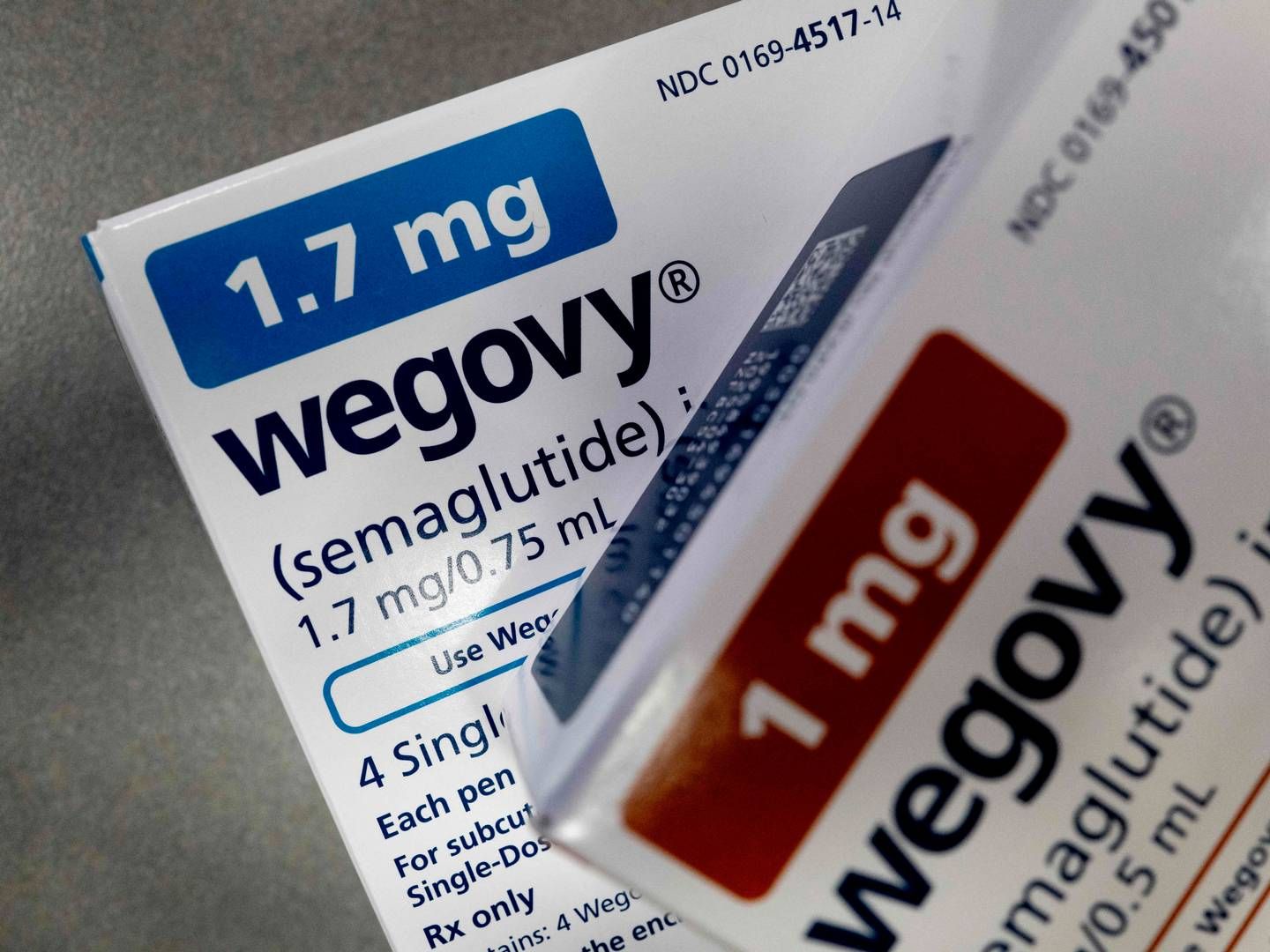
Novo Nordisk within touching distance of European obesity market as CHMP recommends approval

Novo Nordisk has come one step closer to getting its obesity drug Wegovy approved in Europe, as the CHMP has recommended is approval.
Read the whole article
Get access for 14 days for free. No credit card is needed, and you will not be automatically signed up for a paid subscription after the free trial.
With your free trial you get:
Get full access for you and your coworkers
Start a free company trial todayRelated articles
Wegovy weight loss results "very encouraging," says Novo EVP
For subscribers
Raising ambitions at Novo Nordisk an obvious move, says CEO
For subscribers


.jpg)























.jpg&w=384&q=75)




