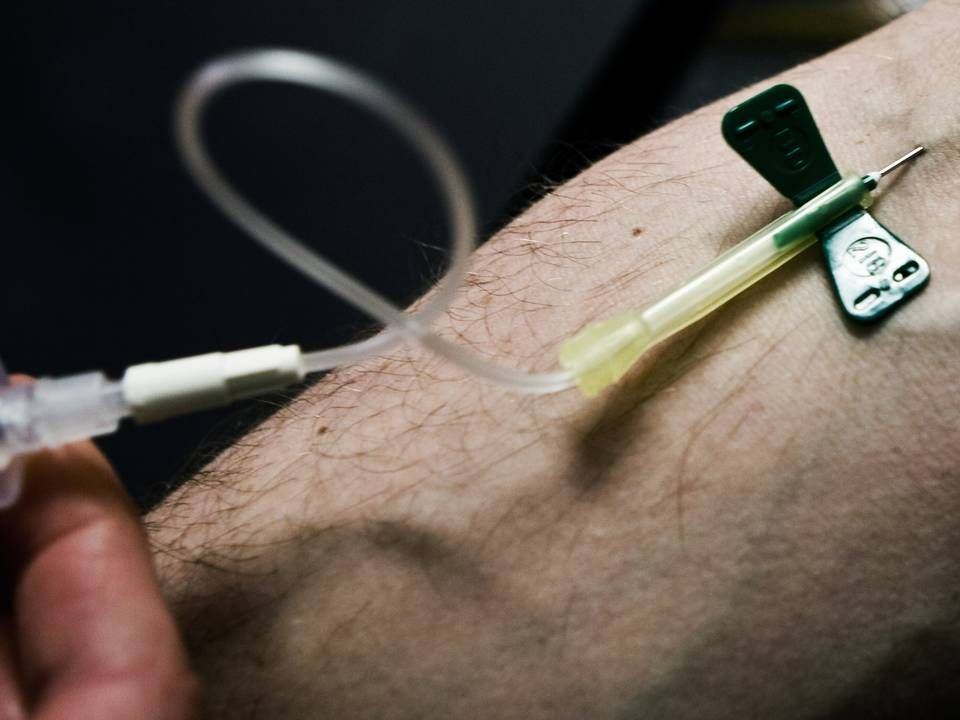
Biomarin is still expecting an EMA decision on hemophilia drug in H1 2022

Biomarin hopes to have a European approval of its potential new drug for the treatment of severe hemophilia A before summer 2022, the company reports in a press release in connection with its third quarter results.
Read the whole article
Get access for 14 days for free. No credit card is needed, and you will not be automatically signed up for a paid subscription after the free trial.
With your free trial you get:
Get full access for you and your coworkers
Start a free company trial todayRelated articles
Biomarin gets ready for launch in second half-year of 2021
For subscribers