US biotech to submit new psoriasis treatment to the FDA in summer
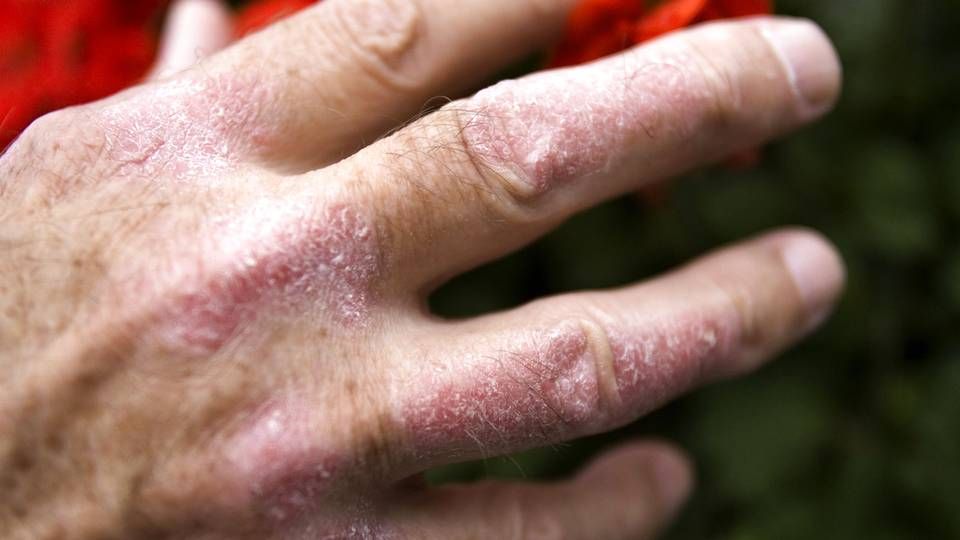
In 2022, US skin doctors may get their hands on a new tool for treating patients with plaque psoriasis.
Read the whole article
Get access for 14 days for free. No credit card is needed, and you will not be automatically signed up for a paid subscription after the free trial.
With your free trial you get:
Get full access for you and your coworkers
Start a free company trial todayRelated articles
Almirall acquires rights to new psoriasis drug in Europe
For subscribers






























